

Inside NURSING.com, we have an entire Lab Values course that covers fluid and electrolytes, acid-base balance, and must know lab values.
Concentration
Concentration is a number that shows the amount of solutes dissolved in a fluid. The word solute is used to describe any substance that is dissolved in a fluid.
Concentration gradient: Molecules in a solution are in a constant state of motion. In a liquid, they will naturally move from areas of higher concentration to areas of lower concentration. A concentration gradient is simply variations in concentrations in a fluid. In the following picture the container on the left has an area to the upper left that has higher concentration than the rest of the container.
Osmolarity: The number of particles in a solution by volume (mOsm/L). It is calculated using osmolality.
Osmolarity: The number of particles in a solution by mass (mOsm/kg). This can be measured using an osmometer. Osmolality is used- more so than osmolarity- in a clinical setting. The osmolality of IV fluids, plasma, urine are used to help paint a picture of volume status in a patient. Osmolality creates osmotic pressure and thus affects movement of water from different compartments in the body.
Some particles in the body can move through membranes easily, while others may need to be transported or assisted with a little pressure. In this section we will discuss different types of movement that occur across body membranes.
Membranes
Different areas of the body are separated by different types of membranes:
Cell membranes: The cell membrane separates cells from the outside environment, intracellular fluid from interstitial or intravascular fluid.
Capillary membranes: The walls of the blood vessels. They separate intravascular fluid from interstitial fluid.
Epithelial membranes: Mucosa of the stomach, intestines, and renal tubules.
The cell membrane contains two layers of phospholipids which have a hydrophilic head (tendency to mix with water) and hydrophobic tail (repelled from water). The hydrophilic tails point toward each other with the hydrophobic heads line each side of the membrane. Embedded in the membranes are different proteins. Because of the nature of the membrane some particles can move freely while others must be transported.
Diffusion: Diffusion occurs when solutes in a solution move from an area of higher concentration to an area of lower concentration. There must be a concentration gradient for diffusion to occur. Diffusion in the body is affected by several different factors: temperature, concentration, size of molecule, and surface area of membrane. Diffusion may also occur due to an electrical gradient. If positively charged ions move into the cell they will be followed by negatively charged ions.
Simple diffusion: Simple diffusion occurs when substances are lipid soluble (oxygen, carbon dioxide) or when they are small enough to travel through protein pores or channels (urea, water). Simple diffusion requires a concentration or electrical gradient.
Facilitated diffusion: Large molecules or molecules that aren’t lipid soluble require facilitated diffusion. Concentration gradients need to be present in facilitated diffusion. Because of its size glucose requires a carrier protein, which allows it to become lipid soluble to move through the cell membrane. Carrier proteins can become saturated if there is a very large difference in concentration between the inside of the cell and the outside.
Active transport: Active transport is required to move molecules against a concentration or chemical gradient. Energy is required for active transport to take place. The sodium-potassium pump is an example of active transport. Carrier proteins can become saturated with excess substrate (molecule upon which an enzyme acts).
To put everything together, the body has many different compartments. Fluids found in each compartment are regulated by membranes, concentrations, and hydrostatic pressure.
Water can move freely from vessels into cells or interstitial spaces. The pressures described above help maintain fluids within the different compartments. The next section describes each of the different areas where fluid is kept and how the different regulatory mechanisms help maintain homeostasis.
The body is made of trillions of cells. Water is found around cells, inside cells, within vessels, and around organs. Each fluid in the body has unique characteristics that allow for the specific functions within each space. The body has many regulatory mechanisms to maintain homeostasis of the fluids.
The different fluids in the body are unique in their electrolyte content. The following chart lists the electrolyte content of different fluids in the body and compares them to the IV fluid that most resembles plasma: lactated ringers.
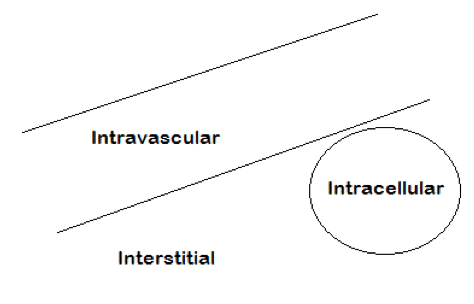
Extracellular fluid: Fluid outside of the cell. ECF includes fluids within the blood vessels (intravascular fluid) and fluid within the interstitial spaces. ECF totals about 15 Liters.
Intravascular Fluid: This includes all the blood within the circulatory system: veins and arteries. IVF, also known as blood contains red blood cells and Plasma. The plasma makes up 3 liters out of 5 liters of IVF.
Interstitial Fluid: Interstitials fluid is found in many compartments throughout the body. Some examples include lymph, synovial fluid, cerebrospinal fluid, digestive secretions, and pericardial fluid. 11-12 liters of fluid.
Intracellular fluid: The fluid components within the cell are the cytoplasm and neoplasm. Intracellular fluids make up 60% of total body water. 25 liters.
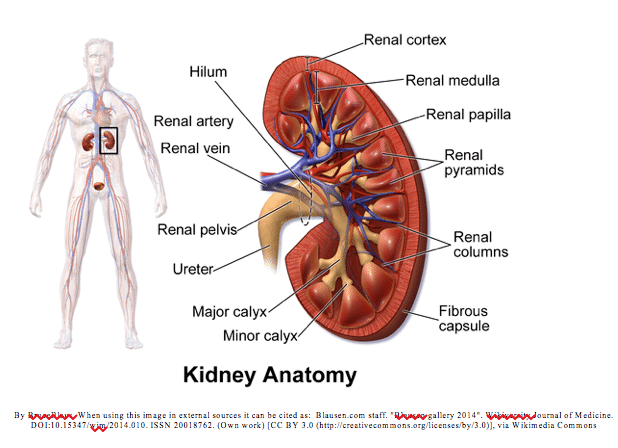
There are two kidneys in the body. They lie on the right and left side of the abdomen below the liver and stomach respectively. They sit toward the back of the abdomen. An adrenal gland sits directly above each bean shaped kidney. A renal artery enters the kidney and the renal vein and ureter exit the kidney. The functional unit of the kidney is the nephron. The nephron helps filter out excess water and solutes from the blood. Filtered blood then leaves via the renal vein, and waste via the ureter.
The kidneys play many vital roles in the body including removal of waste products; regulation of blood pressure and electrolytes; acid-base balance; reabsorption of amino acids, glucose, and water; hormone production: calcitriol, erythropoietin; and enzyme production: renin.
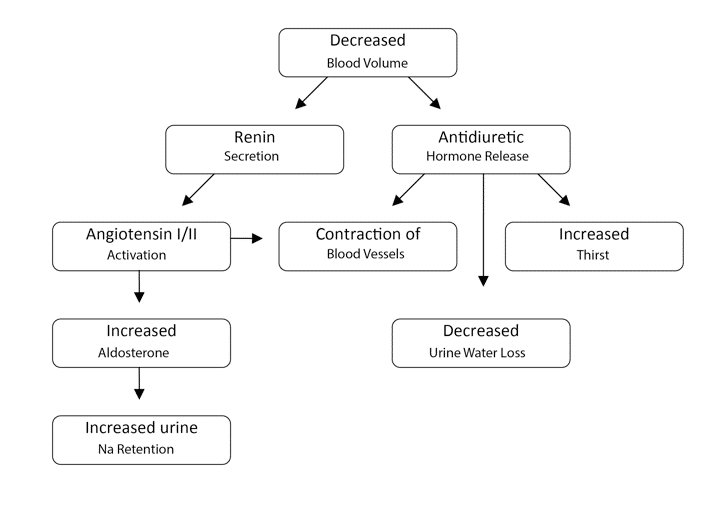
Antidiuretic Hormone (ADH): Stimulated by increased concentration of electrolytes or decreased blood pressure. The hypothalamus has osmoreceptors that monitor osmolarity of the blood. ADH is produced in the hypothalamus and stored in the posterior pituitary gland, it is released from the posterior pituitary into the blood to act on the kidneys. ADH stimulates constriction of blood vessels and water conservation by increasing water reabsorption in the kidneys and decreasing sweat production by sweat glands.
Renin: Created by the kidneys in response to decreased blood flow. Renin stimulates the conversion of angiotensinogen to Angiotensin I, which is then converted to Angiotensin II, via the enzyme angiotensin converting enzyme.
Angiotensin II: Causes blood vessel constriction stimulates aldosterone excretion.
Aldosterone: A hormone made in the kidneys. It works to increase sodium and water reabsorption and increase potassium excretion in urine.
Brain Natriuretic Peptide (BNP): A hormone made in the heart ventricles in response to increase stretching. When stimulated, BNP works to increase sodium and water excretion by the urine. This leads to decreased blood volume and blood pressure.
Atrial Natriuretic Peptide (ANP): A hormone made in the right atrium of the heart in response to increase stretching. Just like BNP, ANP works to increase sodium and water excretion by the urine. This leads to decreased blood volume and blood pressure.
Sodium is the main cation in the blood. Small amounts are also found within the cell.
Sodium concentration is important in maintaining the cell membrane potential. In excitable cells like neurons and muscle cells, membrane potential is essential for communication and muscle contractions respectively. Sodium also helps maintain fluid balance in the blood. It is the main contributor to osmolality of the blood. Sodium contributes 280 mOsm of the 300 mOsm of the blood. Sodium can move into cells, but is pumped out against electrochemical gradient. Na+ absorption is proportional to intake.
Movement from ECF to ICF
If serum sodium increases some sodium will shift into the cell, maintaining the balance of sodium in the blood.
Increase total body sodium
Typical daily intake is usually much higher than our needs.
Excretion
Excretion is regulated by several important hormones: aldosterone, angiotensin II, and natriuretic peptides.
Changes in serum sodium often reflect changes in fluid status. If Na in the blood increases or decreases, the body responds by increasing or decreasing water to maintain sodium concentration. (Remember concentration is affected by both the solute: sodium, and the solvent: water) If you decrease the amount of water you increase concentration. If you increase sodium you increase concentration. Because the body compensates changes in serum sodium concentration are typically caused by changes in water. This makes sodium a good indicator of hydration.
Hyponatremia can be caused by overhydration or body losses of salt water that is replaced with water. The kidneys can compensate be excreting sodium free water. If the body needs to conserve water, however, this compensatory mechanism can’t be used. Hyponatremia causes hypoosmolality since sodium plays such a big role in serum osmolality.
When looking at hyponatremia it is important to know if it is in the setting of decreased, increased, or normal ECF volume. In hyponatremia fluid moves out of the blood and into the interstitial spaces.
The brain has a fixed volume due to the skull. Hyponatremia can lead to increased intracranial pressure and cerebral edema. The body adapts over time by decreasing the concentration within the cells of the central nervous system. Neurologic symptoms are higher in acute hyponatremia versus chronic because of this adaptation. If serum sodium levels get below 120 mMol/L neurological symptoms may be seen. Levels below 115 mMol/L can cause seizures or coma.
**It is dangerous to replete sodium too quickly in chronic hyponatremia. It can cause loss of cerebral fluid due to increase in serum osmolality.
Hyponatremia with increased or normal blood volume
Causes
Signs & Symptoms
muscle weakness, headache, lethargy, apathy, convulsions, confusion, edema, weight gain, elevated BP, muscle cramps, coma, increased mean arterial pressure (MAP), increased, increased central venous pressure (CVP), pulmonary artery pressure (PAP)
Treatment
In hyponatremia that has persisted for more than 48 hours replete sodium slowly or permanent neurologic damage may occur. No more than 24 mmol/L increase in the first 48 hrs.
Hyponatremia with decreased blood volume
Causes
Sign & Symptoms
tremors, personality changes, anxiety, cold skin, irritability, dizziness, postural hypotension, clammy skin, dry mucous membrane seizure, coma, decreased mean arterial pressure (MAP), decreased central venous pressure (CVP), pulmonary artery pressure (PAP), decreased cardiac output, increased systemic vascular resistance
Tests
Treatment
Causes
Signs & Symptoms
During hypernatremia there is decreased tonicity since sodium contributes to osmolality. In response fluid moves out of the cells and into the blood. The cells in the brain adapt by increasing intracellular osmolality. This ensures that the cells won’t lose excess water. In chronic hypernatremia this adaptation has occurs, and symptoms are minimal. As with hyponatremia if serum sodium is adjusted too quickly it can damage the adapted neurons. It could case dangerous cerebral edema.
thirst, fatigue, irritability, altered mental status, coma, fever, flushed skin, peripheral edema, postural hypotension, tachycardia and tachypnea, muscle twitching, deep tendon reflexes
Tests
Treatment
Potassium is the main cation inside the cell. A small amount of potassium is found in the blood and interstitial fluid.
Potassium is transported into the cell via the sodium-potassium pump. Three sodium ions are pumped out of the cell for every two Potassium ions pumped into the cell. The amount of Potassium outside the cell helps maintain the resting membrane potential. Essentially the outside of the cell is more positive and the inside more negative. The electrical charges separated by the cell membrane give the cells in the body a resting membrane potential. In excitable cells like neurons and muscle cells this membrane potential is essential for communication and muscle contractions respectively.
Movement from ICF to ECF
Movement between intracellular fluid (ICF) to extracellular fluid (ECF) is affected by insulin, pH, and epinephrine:
Insulin and epinephrine stimulate glucose uptake in to the cells. They also stimulate activity of the Na-K+ pump. The concentration gradient of sodium that is established by the pump allows for the transport of glucose in to the cell.
The pH affects potassium as well. When pH is low, the excess H+ ion in the blood move into the cells. To maintain electric equilibrium potassium moves out of the cell in response. The opposite happens during alkalosis. It is an inverse relationship, as pH goes down potassium goes up and vise versa.
Increase total body potassium
Tissue breakdown: since the cell is where most potassium is stored, when cells are broken down that potassium is released into the system.
Increased Intake: excess potassium rich foods, salt substitutes, transfusions of whole blood or packed red blood cells.
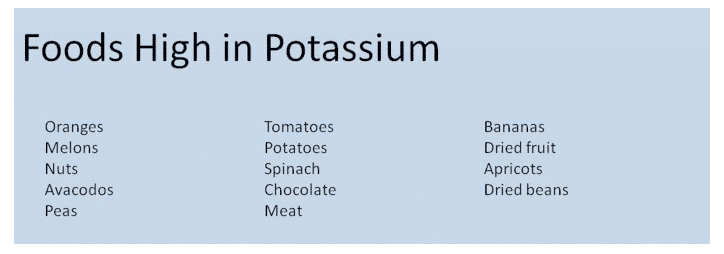
Sources of K+ – fruits, vegetables, beans, dairy, meat
Decreased K+ excretion from the kidneys due to K+ sparing diuretics, renal failure, or Addison’s disease
Excretion
Potassium is excreted via the kidneys-80%, gastrointestinal tract-15%, and the skin-5%.
The kidneys play a big role in potassium regulation. If intake is high, or tissue catabolism occurs the kidneys will quickly compensate and excrete excess serum potassium via the urine. When plasma potassium concentrations are high the adrenal cortex releases aldosterone which will increase excretion of potassium.
Tip: Aldosterone can also be stimulated by postsurgical stress or volume depletion. In these cases you may also see increase potassium excretion. Lastly increased urine production can also cause increase losses of potassium
Mg def: potassium shifts out of cell, and increased K+ excretion occurs.
Causes
Signs & Symptoms
decreased bowel sounds, vomiting, dysrhythmia, muscle weakness, muscle cramps, fatigue, ileus, nausea, constipation, paralysis, hypoventilation, weak pulse, decreased muscle tone

Lab Tests
Treatment
Causes
In DKA potassium in the blood can be elevated despite increased loss of potassium in the urine. During the lack of insulin, acidosis, and increased catabolism potassium moves out of the cells into the blood.
Signs & Symptoms
respiratory distress, diarrhea, irritability, anxiety, muscle weakness, paresthesia, abdominal cramping, anuria, ECG changes, hyperreflexia
Lab Tests
Treatment: medications
Dialysis: removes potassium from the body
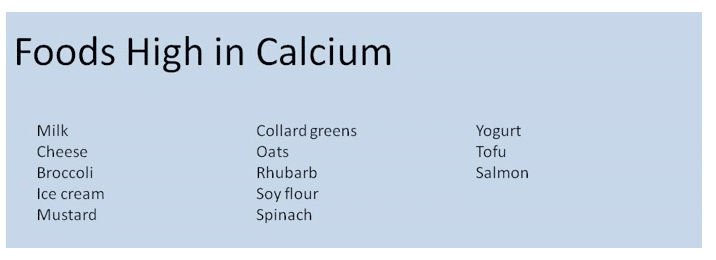
Calcium is stored in the bones and teeth. Calcium Phosphorus salts help give bones their strength. Calcium is found in the ECF but less than 1% of total body Calcium is there. Calcium plays a very important role in muscle contraction, and has an inhibitory affect on neurons.
There are several mechanisms that help maintain the amount of calcium in the blood. When levels of calcium are low parathyroid hormone is released from the parathyroid gland. PTH acts to breakdown bone so that the stored calcium can replete calcium in the ECF. PTH also activates Vitamin D which increase calcium absorption from the intestines. Active Vitamin D also stimulates the kidneys to conserve calcium and excrete phosphorus. When calcium levels are too high the thyroid gland releases calcitonin. Calcitonin stops bone breakdown. Both dietary intake and bone breakdown can lead to increase in calcium levels Calcium is lost in gastrointestinal secretions, urinary excretion, bone deposition, and sweat (in small amounts). Typically we only absorb 20-30% of dietary calcium.
Causes
Since some Calcium in the blood is bound to protein (albumin), when albumin is low total calcium may be low. This is not a problem and doesn’t need treatment as long as ionized Calcium is within normal limits.
Signs & Symptoms
arrhythmias, numbness, tingling fingers, hyperactive reflexes, muscle cramps, tetany, convulsions, tetany, stridor and spasms, lethargy, anxiety, depression, psychosis, decreased heart contractility and heart failure, positive chvostek’s sign and trousseau’s sign
ECG changes: prolonged QT, elongation of ST segment–> ventricle tachycardia.
Tests
Treatment
Causes
Signs & Symptoms
weakness, lethargy, nausea, vomiting, anorexia, polyuria ( from nephrogenic diabetes insipidus), bone pain, fractures, itching, flank pain ( renal calculi), confusion, depression, stupor, coma, personality changes, paresthesia, ECG findings: shortening of ST segment and QT interval, prolonged PR interval. more severe: ventricular dysrhythmia. Risk for digitalis toxicity
Tests
Treatment
Treat underlying cause: partial parathyroidectomy for hyperparathyroidism, chemotherapy for malignant disease, or discontinue ca supplements, vitamin A, vitamin D, thiazide diuretics in renal patients.
IV NS solution: Administer rapidly to increased Ca excretion via urine. Administer diuretics at the same time to prevent volume overload and to increase calcium excretion.
Low calcium diet: limit calcium intake.
Hemodialysis: with low calcium dialysate in renal failure patients.
Phosphorus is the main anion in inside the cell. The majority of Phosphorus is stored with Calcium in bones and teeth – 85%. The inside of the cell contains 14% of phosphorus, and the blood has about 1%. Most phosphorus in the body is in the form of phosphate.
Decrease phosphorus: insulin, glucose, carbohydrate (phosphorus shifts into the cell due to increased needs for of phosphorus during metabolism), alkalosis, specifically respiratory alkalosis due to intracellular shift of phosphorus.
Increase phosphorus: respiratory acidosis can cause shift of phosphorus out of cell, increased intake, intestinal absorption increased, bone reabsorption, impaired renal excretion.
PTH causes increased GI absorption, increased movement of phosphorus out of bone. PTH also increases renal excretion.
Close relationship between calcium and phosphorus.
Causes
Signs & Symptoms
Acute : coma, decreased grip strength, lack of coordination, seizures, chest pain secondary to poor oxygenation, muscle pain, increased risk of infection, confusion, numbness tingling of fingers and circumoral region, difficulty weaning from the vent, decreased strength: difficulty speaking, respiratory muscle weakness (the weakenss in th respiratory muscles can lead to hypoxemia and hyperventilation which may lead to respiratory alkalosis which aggravates the hypophosphatemia), bleeding due to platelet dysfunction, rhabdomyolysis and hemolysis in severe cases.
Chronic: joint pain, weakness, pseudo fractures, lethargy, memory loss, cyanosis, joint stiffness, osteomalacia
Lab Tests
Treatment

Causes
Signs & Symptoms
tetany, muscle weakness, nausea, vomiting, anorexia, tachycardia, hyperreflexia
Most symptoms associated with hyperphosphatemia are actually associated with the accompanying hypocalcemia that often occurs. Other symptoms may occur in metastatic disease due to soft tissue calcification: corneal haziness, conjunctivitis, oliguria, irregular HR, and papular eruptions.
Lab Tests
Treatment: medications
Treat underlying cause: correct volume depletion, treat secondary hyperparathyroidism in chronic renal failure: in chronic renal failure excess PTH can lead to elevated phosphorus and bone disease. Vitamin D can help reduce PTH levels.
Limit Phosphorus intake: avoid high phosphorus foods.
Phosphate binders: (Renvela) Phosphate binders bind to Phosphorus in the GI tract to decrease absorption.
Antacids to bind phosphorus (Aluminum, Calcium, or Magnesium antacids): These medications will bind to phosphorus and decrease absorption. In renal failure Calcium is preferred to Magnesium or Aluminum.
Dialysis: Dialysis may be necessary in severe acute hyperphosphatemia, with symptomatic hypocalcemia.
The majority of magnesium is stored in the bone, 50-60%. Only 1% of magnesium is in the blood, and the remainder is inside the cell. Magnesium activates enzymes that breakdown carbohydrate and protein, triggers the Na/K pump, and plays a role is neuron communication and heart function. Most of Magnesium in the blood is ionized, but there is a portion bound to protein.
Absorption of magnesium is controlled by vitamin D. Excretion is regulated by the kidneys. Excretion is affected by 3 things: excretion of sodium and calcium, blood volume, and parathyroid hormone.
↑ PTH → ↓ Mg Excretion
↓ Sodium and Calcium excretion → ↓ Mg Excretion
↓ blood volume → ↓ Mg Excretion
Causes
Signs & Symptoms
paresthesia, insomnia, loss of appetite, mood changes, confusion, fatigue, weakness, hallucinations
Tests
Treatment

Causes
Signs & Symptoms
sensation of heat, decreased deep tissue reflexes, soft tissue calcification, hypotension, weakness, nausea, vomiting, decreased arterial pressure, bradycardia, lost knee jerk reflex (patellar reflex), diaphoresis, coma, flushing, altered mental function, drowsiness, paralysis, paralysis of respiratory muscles may occur when Mg > 10 mEq/L
Tests
Treatment
Treat underlying cause: if magnesium is high due to medication, d/c the medication (antacids or laxatives that have magnesium: Maalox, Mylanta, Mag Citrate, Milk of Magnesia, Mag-Sulfate)
Diuretics and 0.45% normal saline: will help increase magnesium excretion, as long as patient has adequate renal function.
IV Ca gluconate: counteract neuromuscular effects of Mg if Hypermagnesemia is severe.
Dialysis with a low magnesium dialysate (pt with severe renal impairment)
Hypervolemia is an increase in extracellular fluid (intravascular and interstitial fluid). In hypervolemia the body compensates with the release of natriuretic peptides- which increase excretion of sodium and water by the kidneys- and inhibition of aldosterone.
Causes
Signs & Symptoms
moist skin, edema, ascites, crackles, weight gain, increased blood pressure (decreased BP as heart rate falls), orthopnea, shortness of breath, bounding pulses, wheezes, rhonchi, rales, distended neck veins, tachypnea, tachycardia, gallop rhythm, increased CVP, increased pulmonary artery pressure (PAP), increased pulmonary artery wedge pressure (PAWP), increased mean arterial pressure (MAP) unless pt with heart failure.
Tests
Treatment
Monitor
Hypovolemia is a decrease in intravascular fluid/blood volume. With hypovolemia the body will attempt to compensate by increasing stimulation of the central nervous system: increase heart rate, vascular resistance, thirst, ADH, and aldosterone.
Causes
Signs & Symptoms
sunken eyeballs, increased temp, fatigue, syncope, weight loss, vomiting, increased HR, weakness, constipation, anorexia, thirst, dry tongue, nausea, confusion, oliguria, dizziness, decreased BP, decreased central venous pressure (CVP), decreased pulmonary artery pressure (PAP), decreased cardiac output, decreased mean arterial pressure (MAP), increased systemic vascular resistance
Lab Tests
Treatment: medications
Treatment is based on the type of fluid that is lost. In severe depletion, rapid increase in intravascular fluid is priority.
Treatment with IV Fluids
Crystalloid
Colloid
Medical History:
Look for medical history that might be associated with fluid or electrolyte disturbances
Psychological/ Religious/ Cultural History:
Behavioral, emotional, cultural, socioeconomic, or religious disorders that might be associated with fluid or electrolyte disturbances: bulimia, religious fasting, financial constraints that limit purchase of medications etc.
Hemodynamics
Weight
Taking daily weights is an important indicator of fluid status. Weights should be taken at the same time each day using the same scale if possible. Rapid short term weight changes are a sign of fluid status. A weight change of 1 kg is equivalent to a loss or gain of 1 liter of fluid. Total body water gains could be in any of the body compartments.
I/Os
Fluid status can be monitored by measuring daily intake and output of fluid.
Intake: PO fluids (all drinks and foods that are liquid at room temperature), IV Fluids (exact amounts given should be recorded), irrigation (any irrigation that is not pulled back out should be documented), tube feedings (all administered tube feeds and any water flushes).
Output: urine output, profuse sweating, nasogastric tube suction, draining fistula, rapid breathing liquid stool, wound drainage, vomiting
Body Temp
Elevated temperature leads to increase fluid and electrolyte losses. Fluid volume can also affect temperature: hypovolemia can lead to decreased temperature and vice versa.
Respiratory Rate
Increased respiratory rate leads to increase fluid losses via breathing. Respiratory rate will be elevated in metabolic acidosis. With fluid overload in the lungs crackles or rhonchi may be present.
Heart Rate
Heart rate will increase with decreased fluid volume. In volume overload a bounding pulse is seen. In volume deficit a weak or thready pulse is seen.
Heart rate may be altered with disorders of potassium or magnesium.
Blood Pressure
When blood pressure is decreased it can indicate decrease in fluid volume or possible dysrhythmia from electrolyte abnormalities. Increased blood pressure may indicate increased fluid volume.
Integument System
Cardiovascular System
Neurologic System
Gastrointestinal System
Anorexia, nausea, vomiting, nasogastric suction are all modes of fluid loss. Increased thirst can be a sign of fluid deficit.
Treatment for disorders of fluid balance depends on the cause. It is important to understand the different characteristics of IV fluids available. They are useful in different situations.
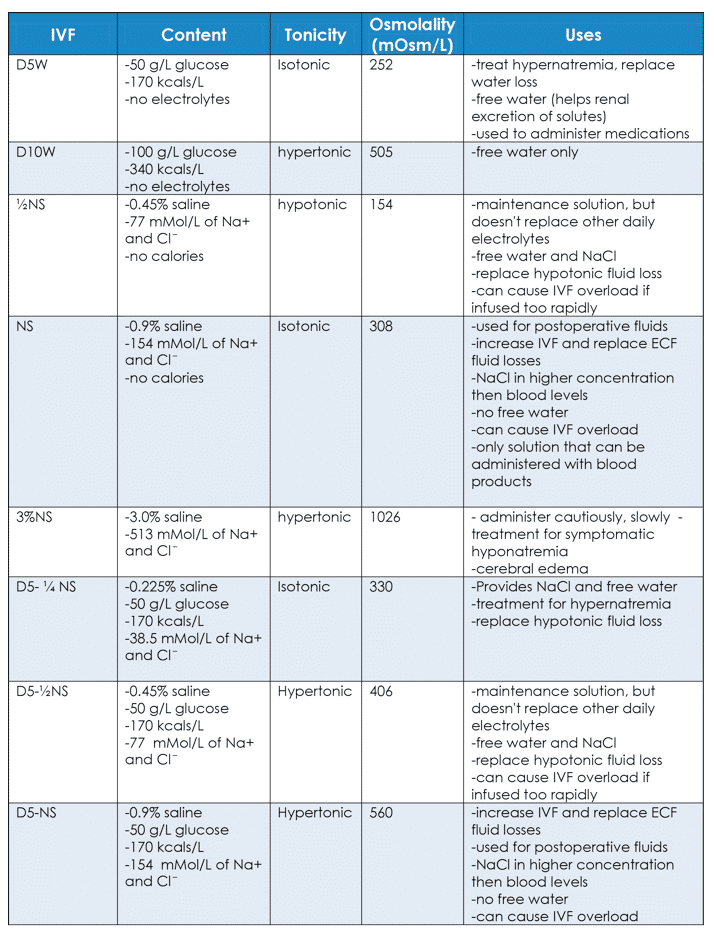
Colloids consist of blood and blood components: blood, packed red blood cells, fresh frozen plasma, plasma, albumin
